Audio By Carbonatix
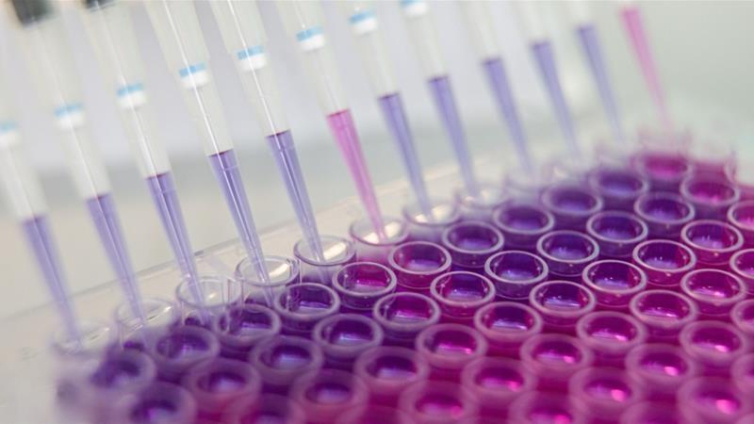
Pfizer Inc. and BioNTech SE said the Covid-19 vaccine they are jointly developing is on track to be submitted for regulatory review as early as October, as they released additional data from an early-stage study.
This global race for an effective vaccine is scary. This is what the news should be coveringhttps://t.co/okqw0SE7CM
— Amiejour (@Beloved_Day1) August 21, 2020
The companies said the vaccine was well tolerated with mild to moderate fever in fewer than 20% of the participants. The companies are continuing to analyze data from the Phase 1 trials in the U.S. and Germany, they said in a statement.
The confirmation of their October goal, first announced last month, helped lift S&P 500 futures briefly on Friday as part of a drumbeat of positive news on inoculation efforts that have the potential to end the threat of damaging lockdowns.
The timeline would make the vaccine one of the fastest-moving in the world. Some analysts expect a vaccine to be approved for use by November in the U.S., a move which may give President Donald Trump a new foothold in the election.
Pfizer and BioNTech last month clinched a $2 billion deal to supply an initial 100 million doses of the vaccine to the U.S. Governments around the world are looking to lock up supplies of still-experimental candidates in hope of stabilizing local economies and stopping spread of the virus that’s taken almost 800,000 lives globally.
The U.S. Food and Drug Administration is tentatively planning an advisory panel to meet Oct. 22 to discuss a Covid-19 vaccine, though it has not specified which vaccines, or how many, will be scrutinized.
Some of the fast-moving efforts around the world have started evaluating efficacy in the final stage of testing, paving the way for regulatory signoff. Russia approved a vaccine dubbed Sputnik V earlier this month, developed by Moscow’s Gamaleya Institute and the sovereign Russian Direct Investment Fund, and plans to administer it widely in October although the shot is still in the midst of human testing. The move has drawn criticism from scientists over concern of mass inoculation being carried out when the drug’s safety profile remains unclear.
Vaccine frontrunners in China have shipped their shots to virus hotspots around the world like Brazil, Indonesia and Saudi Arabia to conduct final-stage trials.
State-owned drugmaker Sinopharm Group Co. said this week that the two inactivated vaccines developed by the company’s subsidiaries will be available as soon as the end of the year and will be priced at less than 1,000 yuan ($145) for two doses. Beijing-based vaccine maker Sinovac Biotech Ltd. expects its shot to win regulatory approval early next year.
Latest Stories
-
GPL 2025/2026: All Blacks hold leaders Medeama at home
6 minutes -
Ghana has over 5 weeks of fuel stock despite Middle East tensions – NPA
9 minutes -
Middle East tensions may hit Ghana’s pumps soon – Duncan Amoah
47 minutes -
WPL 2025/26: Hasaacas beat Army Ladies as Ampem Darkoa Ladies draw
57 minutes -
Five facts about Baba Sadiq, Ghana’s High Commissioner Designate to Nigeria
1 hour -
Baba Sadiq Abdulai appointed as High Commissioner to Nigeria
2 hours -
Playback: The Probe examined Israel-Iran-US tensions and Ghana’s energy security
2 hours -
T-bills auction: Investor appetite remains at all-time high; interest rates tumble to 5.3%
2 hours -
Yes, we “eat Macroeconomics” because it is the foundation of every meal
2 hours -
Annoh-Dompreh launches Nsawam-Adoagyiri Eye Care Project 2026, screens 3,000 residents
2 hours -
British Iranians take to streets of Manchester hours after US-Israeli strikes
3 hours -
Gov’t confirms Black Queens are safe in UAE
3 hours -
Ghana’s Emmanuel Dogbevi re-elected Vice President of African Editors Forum
3 hours -
Three arrested over alleged mob killing of 26-year-old Liberian at Lashibi
3 hours -
African editors chart reform agenda and elect new executive council
3 hours