Audio By Carbonatix
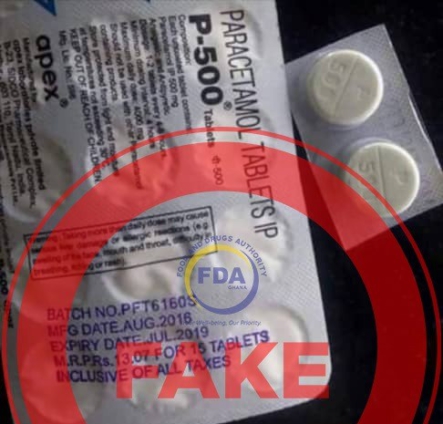
The Food and Drugs Authority (FDA) has dismissed claims circulating on social media that a paracetamol tablet known as “P-500” is on the Ghanaian market.
The said paracetamol is said to be very white and shiny, and it is alleged to have been manufactured by the Indians and Chinese to reduce the population of Africans especially Ghanaians.
But in a statement, the Authority emphasized that the information in circulation is false and must be disregarded.
“FDA has not received any adverse reports of hospitalization associated with the use of any paracetamol tablet.
“The FDA advises that anyone in Ghana who receives the said “alert” should disregard the information and help curb the spread of it; as the content is not true,” he said.
Read full statement below:
PARACETAMOL TABLET- FAKE ALERT
The Food and Drugs Authority (FDA) has noted with concern a recurring “alert” in the form of an audio and image circulating on the various social media platforms stating that a paracetamol tablet, “P-500” is in Ghana.
The tablet is said to be very white and shiny and is alleged to be manufactured by the Indians and Chinese to reduce the population of Africans especially Ghanaians.
The FDA would like to state that, the Authority has not registered and approved for sale any of such paracetamol tablet “P-500” in Ghana.
Further the FDA would like to assure the public that, the content of the circulation cannot be substantiated. In addition the FDA has not received any adverse reports of hospitalization associated with the use of any paracetamol tablet.
The FDA advice that anyone in Ghana who receives the said “alert” should disregard the information and help curb the spread of it; as the content is not true.
Meanwhile, the FDA advises the general public to purchase all medicines from only approved locations or sites, such as the pharmacy, Over the Counter Medicines Sellers and health care facilities.
Kindly check the registration status of regulated products from the FDA website, http://fdaghana.gov.gh, or through the ProPer platform – https://bit.ly/ProPerFDA before purchase.
Latest Stories
-
GACL MD calls for stronger international connectivity to position Accra as West Africa’s aviation hub
54 minutes -
Airlines, travel consultants pledge support for growth at 5th AviationGhana Breakfast Meeting
1 hour -
Mrs Esther Ami Mensah-Abbey, aka Daavi
1 hour -
Mrs Theresa Ata Bosomefi Ayansu
1 hour -
A seat at the table or on the menu? Africa grapples with the new world order
1 hour -
Kenya’s border with Somalia set to re-open after almost 15 years
2 hours -
Second Canada-Africa Agribusiness Summit slated for July 15–16 in Saskatoon
2 hours -
Manchester United fans have say on owner’s immigration claims
2 hours -
Ratcliffe sorry language ‘offended some’ after immigration comments
2 hours -
Trump revokes landmark ruling that greenhouse gases endanger public health
2 hours -
Kim Jong Un chooses teen daughter as heir, says Seoul
2 hours -
Morocco to spend $330m on flood relief plan
2 hours -
Why Ghana’s cocoa has suddenly become expensive
3 hours -
Ghana Progressive Hotels Association raises alarm over high utility tariffs
3 hours -
Ghana’s forest governance gets boost with the EU-funded project launch
3 hours