Audio By Carbonatix
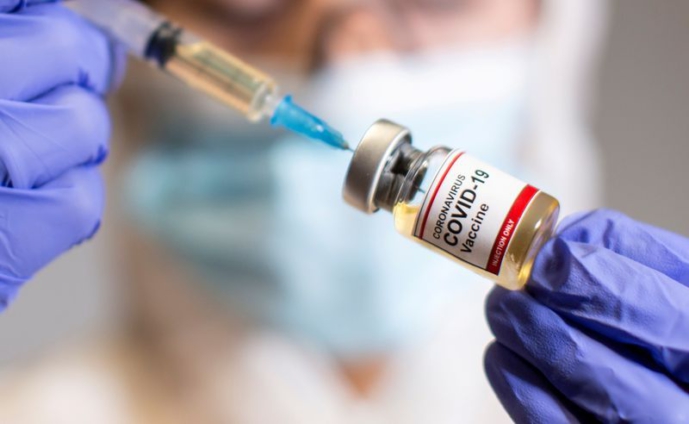
The Food and Drugs Authority (FDA) says it will not permit the administration of unauthorised Stupnik V vaccines that find their way into the country.
In an interaction with JoyNews, Eric Karikari Boateng intimated that the service has put in measures to ensure that the vaccines undergo thorough experiments before they are distributed.
“Of course we can check it out when it gets here to see if there’s a problem with it, the only thing I want to assure Ghanaians is that the FDA will not sit unconcerned for medicines and vaccines that are not of good quality to be administered to people.
“We know the sites that we have approved for the vaccines to come from. So will check to see if it is coming from that side and also if there's the need for further additional checks to see if it’s of quality before it is administered”, he added.
Mr Boateng's comment comes after Brazilian authorities revealed they have suspended the use of the vaccines in their country after they observed serious defects in people who receive the vaccine.
Last week, Government assured it is working assiduously to procure the next batch of AstraZeneca Covid-19 vaccines to inoculate the section of Ghanaians whose second jab is due.
However, there has been a change in plans since India which is the main producer of AstraZeneca doses is currently struggling to cope with demand at home, following a significant surge in cases there.
The head of Laboratory at the FDA indicated that Brazil hasn't announced the components of their vaccine, hence, do not have sufficient evidence to determine if the vaccine is unsafe or not
"For the Brazilians what they were saying is that they found a Lyme virus, you know Stupnik contains two components plus the Ad26 that’s the component one and Ad5 that’s the component two.
"If you look at the specifications, for the Ad2 it tells you that the drug should contain less than 50 with 60 components of the adenovirus per dose of component 2. So I don’t know, that’s why I am saying I have also looked at the Brazilian statement, they have not come out very clearly to tell us if it’s component 2 or not," he stated.
Meanwhile, an Immunologist and Research Fellow at the West Africa Centre for Cell Biology of Infectious Pathogens, University of Ghana, Dr Yaw Bediako has cautioned against the immediate use of Sputnik V vaccines in the country as he says is yet to be approved by any of the large continental bodies.
“In terms of the 61 countries, we have to be very careful because Sputnik V has not been approved by any of the large continental bodies, it’s not been approved by WHO, it’s not been approved by USFDA, it’s not been approved by yet by the European Medical Agency,” he said.
Latest Stories
-
Ablakwa secures rare access to Ghana’s 2 prisoners of war in Ukraine, pushes for their release
29 minutes -
Today’s Front pages: Friday, February 27, 2026
40 minutes -
Premier League: Arsenal v Chelsea preview
49 minutes -
Ghana loses over GHS 6.2bn annually to poor sanitation – ISSER study warns
1 hour -
Prudential Bank marks February with distribution of Ghanaian chocolate to customers
2 hours -
KMA finally elects Presiding Member after stalemate
3 hours -
Nana B rallies Ayawaso East voters to back NPP’s Baba Ali in March 3 by-election
3 hours -
Be honest with Ghanaians on gold policy – Oppong Nkrumah to gov’t
3 hours -
Lands Minister refutes claims of missing seized excavators, unveils tracking system
3 hours -
Ghana set to launch National AI Strategy to boost local innovation – Sam George
3 hours -
PURC gives ECG 48 hours to fix prepaid metering concerns
3 hours -
Makola No. 2 Market managers justify rent increase amid traders’ protests
3 hours -
Mahama to deliver 2026 State of the Nation Address today
3 hours -
Rapid prepaid electricity depletion not caused by smart meters – Adomako-Mensah rejects ECG’s assertion
3 hours -
GoldBod warns licence holders over failure to file monthly gold transaction reports
3 hours