Audio By Carbonatix
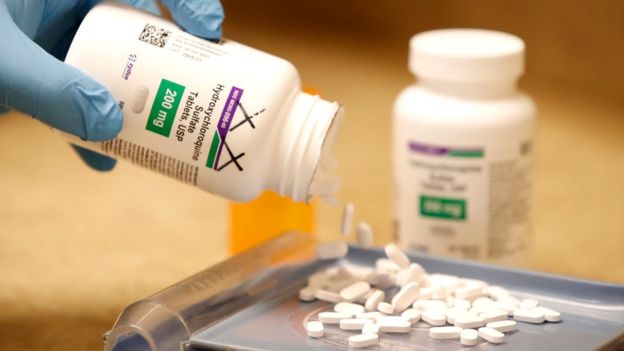
Testing of the malaria drug hydroxychloroquine as a possible treatment for coronavirus has been halted because of safety fears, the World Health Organization (WHO) says.
Trials in several countries are being "temporarily" suspended as a precaution, the agency said on Monday.
It comes after a recent medical study suggested the drug could increase the risk of patients dying from Covid-19.
President Donald Trump has said he is taking the drug to ward off the virus.
The US president has repeatedly promoted the anti-malarial drug, against medical advice and despite warnings from public health officials that it could cause heart problems.
Last week, a study in medical journal The Lancet said there were no benefits to treating coronavirus patients with hydroxychloroquine, and that taking it might even increase the number of deaths among those in hospital with the disease.
Hydroxychloroquine is safe for malaria, and conditions like lupus or arthritis, but no clinical trials have recommended its use for treating Covid-19.
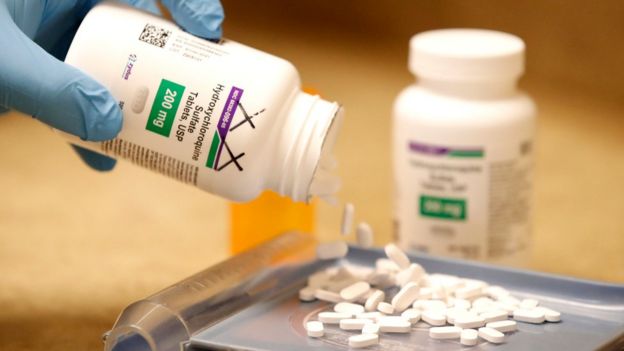
The WHO, which is running clinical trials of various drugs to assess which might be beneficial in treating the disease, has previously raised concerns over reports of individuals self-medicating and causing themselves serious harm.
On Monday, officials at the UN health agency said hydroxychloroquine would be removed from those trials pending a safety assessment.
The Lancet study involved 96,000 coronavirus patients, nearly 15,000 of whom were given hydroxychloroquine - or a related form chloroquine - either alone or with an antibiotic.
The study found that the patients were more likely to die in hospital and develop heart rhythm complications than other Covid patients in a comparison group.
The death rates of the treated groups were: hydroxychloroquine 18%; chloroquine 16.4%; control group 9%. Those treated with hydroxychloroquine or chloroquine in combination with antibiotics had an even higher death rate.
The researchers warned that hydroxychloroquine should not be used outside of clinical trials.
Latest Stories
-
Hamamat and Wiyaala land tourism ambassadorial roles
1 hour -
A singer’s tragic death highlights Nigeria’s snakebite problem
2 hours -
King Charles to host Nigeria’s first UK state visit in 37 years
3 hours -
Mikel Arteta: Arsenal’s 9-point lead at top of Premier League means ‘nothing’
3 hours -
Japan votes in snap election as PM Takaichi takes a gamble
4 hours -
Bloodshed in Kpandai as rival chieftaincy factions clash over gravel pit
5 hours -
Most couples learn these 12 hard lessons way too late
5 hours -
Vote-buying allegations: Refer Ayawaso East incident to OSP — Mussa Dankwah tells Mahama
5 hours -
Government plots audacious 180,000-hectare coconut expansion to dominate global markets
6 hours -
AMA doubles sweepers’ wages to GH₵800
7 hours -
Ashie Moore admits defeat in war against vote buying
7 hours -
UniMAC mourns with family as student killed in road crash is laid to rest
8 hours -
Bribery scandal rocks NDC Ayawaso East primary as IMANI President demands total annulment
8 hours -
Pollster Mussa Dankwah reacts as Baba Jamal defies projections in NDC Ayawaso East Primary
8 hours -
Government to roll out Free Primary Healthcare in the first week of April
9 hours