
Audio By Carbonatix
The Food and Drugs Authority (FDA) has ordered an immediate suspension of the manufacture of Tasty Tom Enriched Tomato Mix, citing serious health and safety breaches at the production facility of Nutrifoods Ghana Limited.
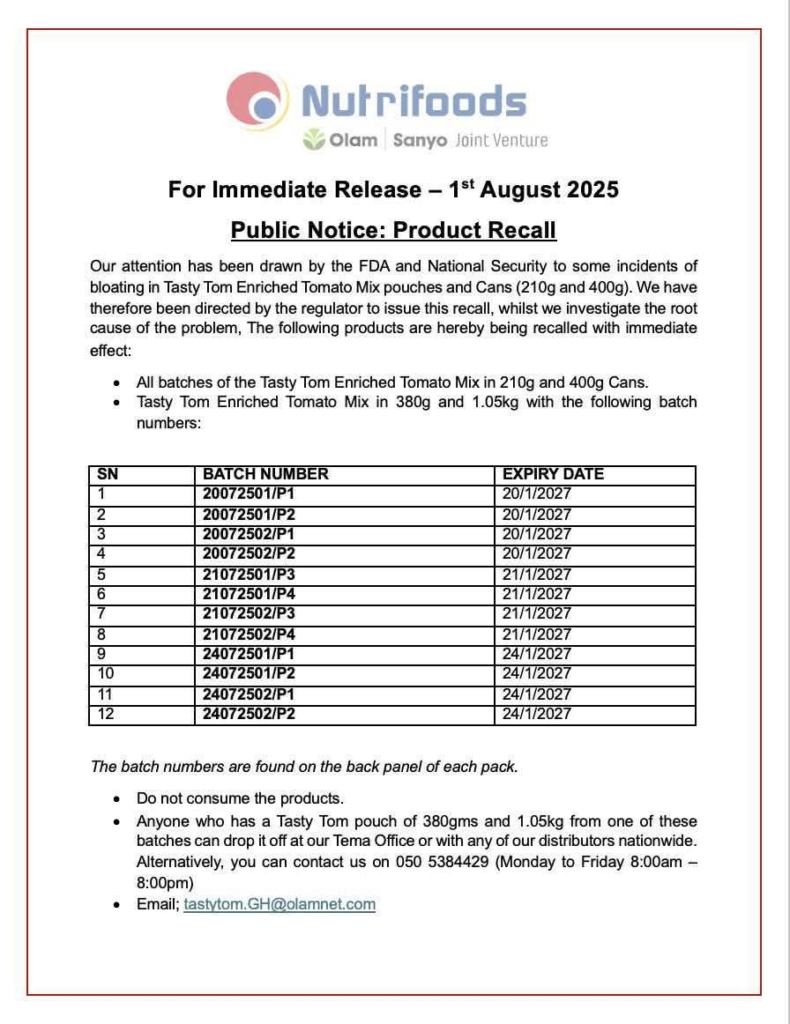
The decision, according to a press statement issued on Sunday, August 3, follows an earlier directive for the recall of all canned Tasty Tom Enriched Tomato Mix products, as well as specified batches of the product in 380g and 1.05kg pouches.
The FDA's action was prompted by numerous consumer complaints and a subsequent investigation into the company's operations.
Findings from the FDA inspection revealed poor maintenance of key manufacturing equipment and a lack of adequate monitoring systems to ensure product safety.



These lapses were found to compromise the integrity of the tomato mix, especially in canned variants where faulty sealing mechanisms led to contamination.
Several pouches were reported to be bloated, and in some instances, mould was discovered, posing serious risks to consumer health.
The FDA emphasised the gravity of the situation: “These breaches present unacceptable risks to public health. We have acted swiftly to suspend production and ensure the affected products are removed from the market.”
The Authority also disclosed that Nutrifoods had previously been barred from manufacturing the tomato mix in January 2025, raising questions about compliance and regulatory enforcement.
As a result, the FDA has launched an internal probe to determine whether any lapses occurred within its regulatory oversight.
“We are committed to transparency and accountability. Should any internal failings be identified, decisive action will be taken to strengthen our regulatory framework,” the FDA assured.
According to the statement, retailers, wholesalers, and the general public are advised to take immediate note of this directive and cooperate fully with the ongoing recall.
Consumers in possession of the affected products are encouraged to return them to their point of purchase and report any adverse effects experienced.
Nutrifoods has, in a communique, agreed to recall products with the specified batch numbers as directed by the FDA.
Latest Stories
-
Woman found dead at Dzodze
4 minutes -
Nana Aba Anamoah rates Mahama’s performance
21 minutes -
Ghana selects Bryant University as World Cup base camp
1 hour -
Nana Aba Anamoah names Doreen Andoh and Kwasi Twum as her dream interviewees
1 hour -
Religious Affairs Minister urges Christians to embrace charity and humility as Lent begins
3 hours -
Religious Affairs Minister calls for unity as Ramadan begins
3 hours -
Willie Colón, trombonist who pioneered salsa music, dies aged 75
3 hours -
Ga Mantse discharged from UGMC following Oti Region accident
4 hours -
Guardiola tells team to chill with cocktails as Man City pile pressure on Arsenal
4 hours -
Majority blasts Minority over Burkinabe border bloodbath claims
5 hours -
Analyst says Burkina Faso killings were a calculated signal to Ghana
6 hours -
Veep extends Ramadan greetings, donates to Cape Coast Central Mosque
7 hours -
Watch the moment President Mahama visited the Ga Mantse at UGMC after horrific accident
7 hours -
UBIDS secures $6.6m prefabricated classroom complex to end space deficit
8 hours -
Gold Fields Ghana Foundation deepens childhood cancer awareness drive; invests $4.8m in community health
9 hours

