
Audio By Carbonatix
The Food and Drugs Authority (FDA) has ordered an immediate suspension of the manufacture of Tasty Tom Enriched Tomato Mix, citing serious health and safety breaches at the production facility of Nutrifoods Ghana Limited.
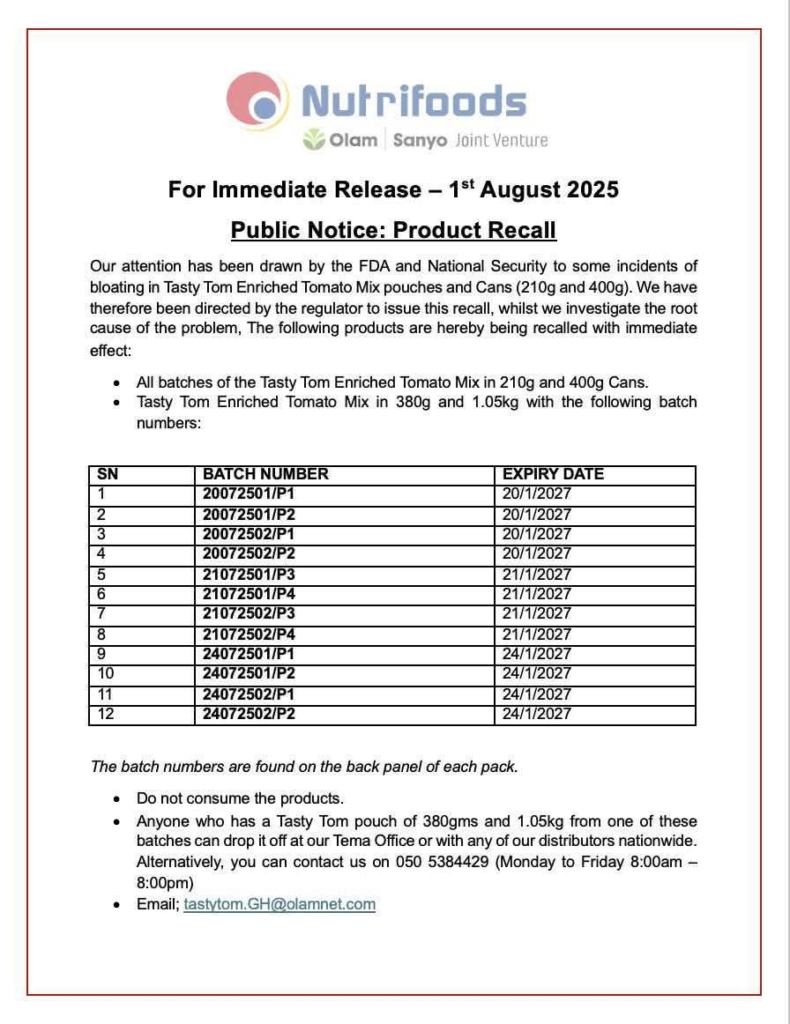
The decision, according to a press statement issued on Sunday, August 3, follows an earlier directive for the recall of all canned Tasty Tom Enriched Tomato Mix products, as well as specified batches of the product in 380g and 1.05kg pouches.
The FDA's action was prompted by numerous consumer complaints and a subsequent investigation into the company's operations.
Findings from the FDA inspection revealed poor maintenance of key manufacturing equipment and a lack of adequate monitoring systems to ensure product safety.



These lapses were found to compromise the integrity of the tomato mix, especially in canned variants where faulty sealing mechanisms led to contamination.
Several pouches were reported to be bloated, and in some instances, mould was discovered, posing serious risks to consumer health.
The FDA emphasised the gravity of the situation: “These breaches present unacceptable risks to public health. We have acted swiftly to suspend production and ensure the affected products are removed from the market.”
The Authority also disclosed that Nutrifoods had previously been barred from manufacturing the tomato mix in January 2025, raising questions about compliance and regulatory enforcement.
As a result, the FDA has launched an internal probe to determine whether any lapses occurred within its regulatory oversight.
“We are committed to transparency and accountability. Should any internal failings be identified, decisive action will be taken to strengthen our regulatory framework,” the FDA assured.
According to the statement, retailers, wholesalers, and the general public are advised to take immediate note of this directive and cooperate fully with the ongoing recall.
Consumers in possession of the affected products are encouraged to return them to their point of purchase and report any adverse effects experienced.
Nutrifoods has, in a communique, agreed to recall products with the specified batch numbers as directed by the FDA.
Latest Stories
-
Africa’s top editors converge in Nairobi to tackle media’s toughest challenges
46 minutes -
Specialised courts, afternoon sittings to tackle case delays- Judicial Secretary
49 minutes -
Specialised high court division to be staffed with trained Judges from court of appeal — Judicial Secretary
1 hour -
Special courts will deliver faster, fairer justice — Judicial Secretary
2 hours -
A decade of dance and a bold 10K dream as Vivies Academy marks 10 years
2 hours -
GCB’s Linus Kumi: Partnership with Ghana Sports Fund focused on building enduring systems
3 hours -
Sports is preventive healthcare and a wealth engine for Ghana – Dr David Kofi Wuaku
3 hours -
Ghana Sports Fund Deputy Administrator applauds GCB’s practical training for staff
3 hours -
Ghana Sports Fund strengthens institutional framework with GCB Bank strategic partnership
3 hours -
UBIDS to Complete Abandoned Projects Following GETFund Financial Clearance – Vice Chancellor
3 hours -
Nii Moi Thompson questions Anokye Frimpong’s ‘distorted history’ narratives
4 hours -
Anthony O’Neal set to receive Ghanaian citizenship, prepares to launch ‘Class on the Bus’ Initiative
4 hours -
South Tongu MP inspects GH₵500,000 surgical equipment, supports District Court with logistics
5 hours -
Kpasec 2003 Year Group hosts garden party to rekindle bonds and inspire legacy giving
7 hours -
Financing barriers slowing microgrid expansion in Ghana -Energy Minister
7 hours

