Audio By Carbonatix
The World Health Organization has said that a batch of contaminated India-made cough syrup has been found in the Marshall Islands and Micronesia.
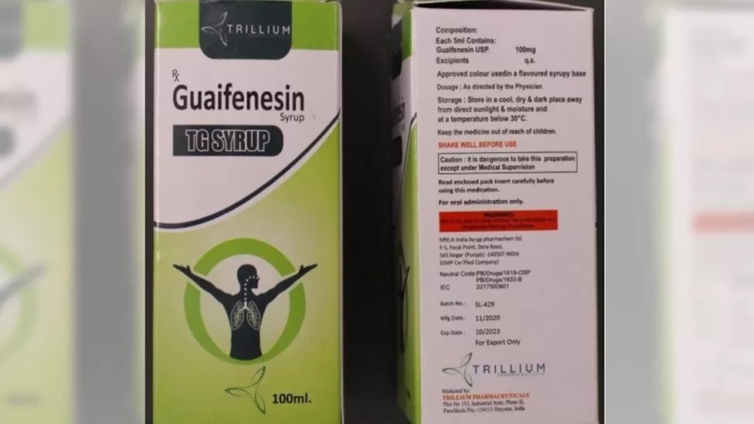
The WHO said that the tested samples of Guaifenesin TG syrup, made by Punjab-based QP Pharmachem Ltd, showed "unacceptable amounts of diethylene glycol and ethylene glycol".
Both compounds are toxic to humans and could be fatal if consumed.
The WHO statement did not specify if anyone had fallen ill.
The latest alert comes months after the WHO linked other cough syrups made in India to child deaths in The Gambia and Uzbekistan.
Sudhir Pathak, managing director of QP Pharmachem, told the BBC that the company had exported the batch of 18,346 bottles to Cambodia after getting all due regulatory permissions. He said he didn't know how the product had reached the Marshall Islands and Micronesia.
"We did not send these bottles to the Pacific region, and they were not certified for use there. We don't know under what circumstances and conditions these bottles reached the Marshall Islands and Micronesia," he said, adding that his company has sent a legal notice to the firm that exported the batch of medicines to Cambodia.
The WHO statement said that the product, which is used to relieve chest congestion and cough symptoms, was tested by Australia's drug regulator, the Therapeutic Goods Administration.
The syrup was marketed by Trillium Pharma, based in Haryana state. The BBC couldn't reach a Trillium representative on the phone. The Indian government has not reacted to the latest alert.
The statement added that "neither the stated manufacturer nor the marketer have provided guarantees to WHO on the safety and quality of these products".
India is the world's largest exporter of generic drugs, meeting much of the medical needs of developing countries.
But in recent months, many Indian firms have come under scrutiny for the quality of their drugs, with experts raising concerns about the manufacturing practices used to make these medicines.
In October, WHO had sounded a global alert and linked four cough syrups made by Maiden Pharmaceuticals to the deaths of 66 children from kidney injuries in The Gambia.
Both the Indian government and the company, Maiden Pharmaceuticals, had denied the allegations.
In March, India cancelled the manufacturing licence of a firm whose cough syrups were linked to 18 child deaths in Uzbekistan. Earlier this month, the FDA said it had found that the Indian manufacturer of eye drops linked to three deaths and serious infections in the US had violated several safety norms.
Latest Stories
-
Mz Nana, other gospel artistes lead worship at celebration of life for Eno Baatanpa Foundation CEO
49 minutes -
Ayawaso East NDC Primary: Baba Jamal campaign distributes TV sets, food to delegates
1 hour -
MzNana & Obaapa Christy unite on soul-stirring gospel anthem Ahoto’
1 hour -
Ayawaso East by-election underway as five candidates vie for NDC ticket
2 hours -
Loyalty is everything in politics; Bawumia must decide on Afenyo-Markin – Adom-Otchere
2 hours -
Ghana positions itself as a Competitive Fund Domiciliation Hub
2 hours -
NPP: Afenyo-Markin defends post-election coordination, urges focus on party unity
2 hours -
MoMo boss Shaibu Haruna named fintech CEO of the Year as MobileMoney Ltd, MTN Ghana sweep top awards
3 hours -
Kofi Bentil praises Afenyo-Markin’s leadership style but calls it combative
3 hours -
NDC’s demolishing exercises will feature in 2028 election – Adom Otchere
3 hours -
“I was hoping for 60%” – Paul Adom-Otchere on Dr Bawumia’s flagbearer win
4 hours -
Africa’s growth depends on empowering SMEs, women and youth – CEO of Telecel Group
4 hours -
Force for good in action: Absa’s colleague volunteerism in 2025
4 hours -
14-Year-old boy drowns at Fiapre Catholic Junction in Bono Region
4 hours -
KIA too big to be named after Kotoka – Kofi Bentil
4 hours