The Oxford coronavirus vaccine trial is facing a "challenge", the health secretary has admitted, after it was put on hold due to a suspected serious adverse reaction in one of its volunteers.
Researchers have paused the trial while they investigate the reaction in one of the participants in the UK, it was announced on Tuesday night.
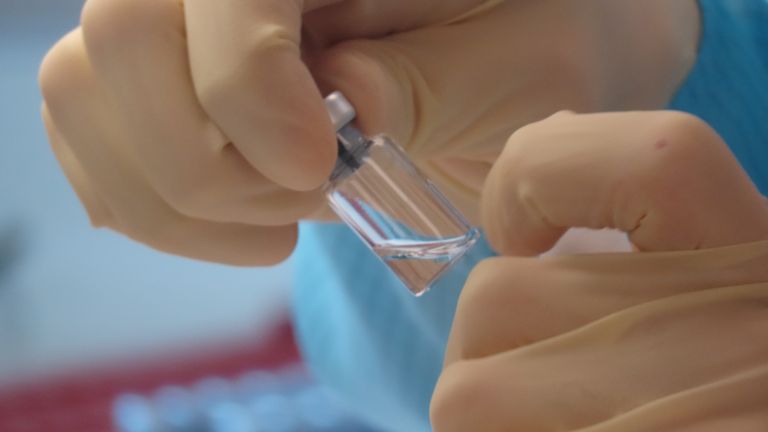
"As part of the ongoing randomised, controlled global trials of the Oxford coronavirus vaccine, our standard review process was triggered and we voluntarily paused vaccination to allow the review of safety data by an independent committee," a spokesperson for AstraZeneca - the drugmaker working with Oxford University - said.
They explained it was a "routine action" and that it is speeding up the investigation to minimise any potential impact on the trial's timeline.
"We are committed to the safety of our participants and the highest standards of conduct in our trials," they added.
Health Secretary Matt Hancock told Sky News' Kay Burley programme the pause is not necessarily caused for concern and that it has already overcome one such delay.
"It is obviously a challenge to this particular vaccine," he says.
"It's not actually the first time it has happened to the Oxford vaccine and it's a standard process in clinical trials."
Asked if it is a setback, Mr Hancock replied: "Not necessarily, it depends on what they find when they do the investigation.
"There was a pause earlier in the summer and that was resolved without a problem."
The nature of the adverse reaction and when it happened are not currently known.
Clinical holds usually mean there is a pause in recruiting new participants and dosing current ones.
It is not uncommon for trials to be put on hold, but scientists are under pressure to develop a vaccine to help curb the pandemic.
Most serious adverse reactions that occur after vaccination are not related to the injection and are coincidental health problems, the World Health Organisation (WHO) has said.
The Director General of the World Health Organisation boss, WARNS leaders of countries NOT TO LISTEN to ‘vaccination concerns’ from their citizens, and just follow ‘UN orders’ to vaccinate their entire population! https://t.co/WWK5oPrLsG
— BeachMilk (@YellowCube7) August 25, 2020
When a vaccine is given to a large number of people, it is likely that a few people will experience a medical problem around the time of vaccination - but this does not prove any cause and effect.
Even so, researchers will need to investigate if there is any link.
In July, early results from the trial showed the vaccine was safe and produced strong immune responses in volunteers.
No unexpected adverse reactions were recorded at the time, although more than half of 1,000 participants reported mild or moderate side effects including fever, headaches, muscle pain and soreness at the injection site.

Phase three trials of the Oxford vaccine had recently expanded to the US, recruiting up to 30,000 adults.
Trials were also underway in South Africa and Brazil.
Experts believe finding a vaccine is the only way for the world to return to normal in the future and there are currently nine vaccine candidates in larger phase three trials.
But it is not known how well a vaccine will work, and the top US infectious diseases expert Dr Anthony Fauci recently warned the chances of it being almost 100% effective are "not great".
"We don't know yet what the efficacy might be. We don't know if it will be 50% or 60%. I'd like it to be 75% or more," he said.
Latest Stories
-
Akesse Brempong to hold ‘Davidic’ concert on April 21
27 mins -
Here’s why Dr. Steve Manteaw supports Otumfuo’s call for ECG, GRIDCo divestiture
41 mins -
Ejisu by-election: Former NPP MP files nomination to contest as independent candidate
1 hour -
Aklerh out with ‘Dancehall Queen’ EP
1 hour -
Burna Boy makes Time Magazine’s 100 Most Influential People
1 hour -
National Peace Council cautions clergy against spiteful comments ahead of December 7 polls
1 hour -
My wife sent nudes to her Dubai lover when I was battling kidney disease – Erico
2 hours -
Kudus urges West Ham to eye Ghanaian youngster Emmanuel Danso
2 hours -
Don’t be cowed into silence, defend Ghana’s democracy – Sam Jonah tells journalists
2 hours -
QNET triumphs at PR Awards 2024 with 3 prestigious wins
2 hours -
Dubai Airport slowly re-opening as UAE rainfall persists
2 hours -
Dumsor: Kumasi demonstrators demand immediate release of load-shedding timetable
2 hours -
Transport operators frustrated by long wait to hike fares
2 hours -
MTN FA Cup: Dreams FC’s quarterfinal game against Soccer Intellectuals set for May 1
2 hours -
Enimil Ashon: Season of promises and scholarship for the greedy
2 hours

